The blisters and sachets of medicines that are part of our daily life are the result of a fascinating process known as pharmaceutical freeze-drying.
But why is freeze drying so important in the production and storage of medicines? And which parameters must be constantly monitored during the lyophilization of a drug? That's what we're going to find out in this article.
What freeze-drying is
The goal of the freeze-drying process is to eliminate water from a compound without affecting its chemical and physical properties.
The freeze-dried products, being completely free of water molecules, can be stored for a very long time in the correct conditions.
Thanks to freeze-drying it is possible to preserve not only drugs, but also plasma, bacteria, proteins and biological tissues.
3 stages of freeze-drying
Despite the variety of pharmaceutical applications, the product to be lyophilized always goes through 3 stages:
- Freezing. The product is brought to very low temperatures, even several tens of degrees below zero, with ambient atmospheric pressure (which means about 1000 millibar).
- Pressure decreasing. Air is removed from the freeze dryer, reaching very low atmospheric pressures, in the range of a few millibars. At this pressure, the frozen water does not pass into a liquid state, but it sublimates, becoming steam that will be expelled from the process.
- Drying. The temperature is gradually increased, favouring the sublimation process of the last particles of water.
Freeze-drying process: what physical quantities need to be measured
During freeze-drying, two parameters are involved:
1. Temperature. The temperature can be monitored through the use of data loggers that allow you to measure temperatures below zero, such as S-MicroW L Ultra Freeze.
2. Pressure. The air inside a pharmaceutical freeze dryer is almost completely eliminated. Therefore, pressures in the range of a few millibars can be reached. The best data logger in these cases is certainly the Pirani Vacuum Logger, based on the innovative Pirani Vacuum Meter.
The physical basis of the Pirani Vacuum Logger
The Pirani Vacuum Logger gives the possibility to measure extremely low pressures with very high precision, in the range of one ten thousandth of a bar.
To understand how the Pirani Vacuum Logger works, we need to start from the small quantities world.
The physics of heat
We are used to think of molecules as static objects linked together. In reality they are never still, but they vibrate. The movement of the molecules releases energy in heat form. Consequently, the more the molecules of an object vibrate, the hotter the object will be.
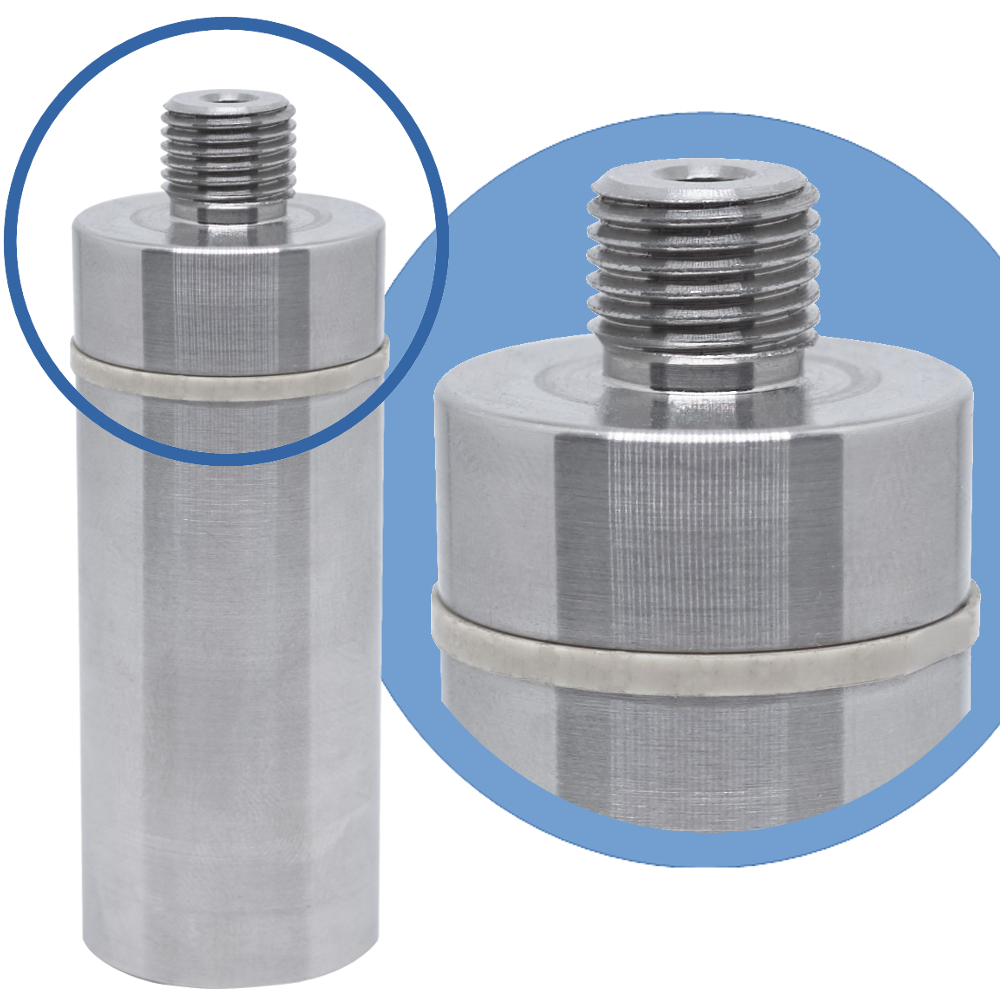
And here it is the Pirani vacuum gauge
Inside the Pirani vacuum gauge there is a metal wire that runs on electricity that heats it. The colder air molecules come into contact with the wire.
As a result, the air molecules acquire some of the vibration, while the wire loses it. This means that the air temperature increases, while that of the wire decreases.
The more air molecules there are, the more contact with the wire molecules will occur. Therefore the heat exchange will be faster.
If we reduce the number of air molecules, we will have less contact between them and the molecules of the wire. So the wire will take longer to change its temperature.
And then If we could completely or almost completely remove the air molecules, no more heat exchanges would take place, since the wire would not find molecules to which it could transfer its temperature.
So far the system is perfect. But the initial problem remains unsolved…
How is vacuum measured?
To answer this question, let's go back to our electric current-powered wire. Now that we have removed most of the air molecules, the molecules of the wire have no longer the ability to release heat, and therefore the temperature of the wire continues to increase.
As the temperature increases, the electrical resistance also increases. This is the turning point. But we cannot measure this value directly.
The resistance is obtained by applying the formula of Ohm's law, in which
V=RI
The voltage (V) is equal to the product of the resistance (R) times the electric current (I)
We can easily measure the tension from one end of the wire to the other. Furthermore, we already know the intensity of the electric current that passes through it. Therefore we can get the value of the electrical resistance by applying a simple algebraic expression.
Once we know the electrical resistance of the wire, we can very accurately calculate the "amount of vacuum" in which our wire is immersed.
The Pirani Vacuum Logger advantages
- It can detect pressures with a very high resolution, impossible to obtain with other pressure sensors.
- It can record even in extreme vacuum conditions.
- It withstands very wide temperature fluctuations: from 85 ° C to -60 ° C.
- It requires very little electricity. This is why the batteries have a very long life.
Furthermore, our Pirani Vacuum Logger is equipped with a ½ Gass thread to screw it onto the autoclave.
A real case of Pirani Vacuum Logger application for pharmaceutical freeze-drying
One of our clients active in the pharmaceutical validation field needed to obtain very precise data that indicates the success of the whole process. Initially, to validate the freeze drying cabinets, he installed our S-MicroW L Ultra Freeze logger to measure cryogenic temperatures. Despite this, there were still critical points in the process.
In order to investigate any further and understand where the problem was to improve the final result of the procedure, we have implemented in the monitoring system some Pirani Vacuum Loggers together with temperature loggers dedicated to ultra freezers.
With the data obtained, the customer was able to recalibrate the process, obtaining very accurate and reliable results from the freeze drying process.
 Flip through our history
Flip through our history